Answer :
The mass of perchloric acid is, 26.5 grams.
The mass of water in the same solution is, 11.1 grams
Explanation :
As we are given that 70.5 wt % aqueous perchloric acid that means 70.5 grams of perchloric acid present in 100 grams of solution.
Now we have to determine the mass of perchloric acid in 37.6 grams of aqueous perchloric acid.
As, 100 grams of aqueous perchloric acid (solution) contains 70.5 grams of perchloric acid.
So, 37.6 grams of aqueous perchloric acid (solution) contains
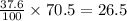 grams of perchloric acid.
grams of perchloric acid.
Thus, the mass of perchloric acid is, 26.5 grams.
Now we have to determine the mass of water are in the same solution.
Total mass of solution = 37.6 g
Mass of perchloric acid = 26.5 g
Mass of water = Total mass of solution - Mass of perchloric acid
Mass of water = 37.6 g - 26.5 g
Mass of water = 11.1 g
Thus, the mass of water in the same solution is, 11.1 grams