According to Ideal gasTo solve this problem, the fastest relationship allows us to observe the proportionality between the two variables would be the one expressed in the ideal gas equation, which is
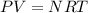
Here
P = Pressure
V = Volume
N = Number of moles
R = Gas constant
T = Temperature
We can see that the pressure is proportional to the temperature, then
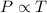
This relationship can be extrapolated to all the scenarios in which these two variables are related. As the pressure increases the temperature increases. The same goes for the pressure in the atmosphere, for which an increase in this will generate an increase in temperature. This variable can be observed in areas of different altitude. At higher altitude lower atmospheric pressure and lower temperature.