Answer:
6.66 s will it take for [AB] to reach 1/3 of its initial concentration 1.50 mol/L.
Step-by-step explanation:
![Rate = k[AB]^2](https://img.qammunity.org/2021/formulas/chemistry/college/kc3hqsfzqw6hw2q4kpt032w0wfe7b16bti.png)
The order of the reaction is 2.
Integrated rate law for second order kinetic is:
![(1)/([A_t]) = (1)/([A]_0)+kt](https://img.qammunity.org/2021/formulas/chemistry/college/bj32u0ycxy039bxo2npb8w4kujg1ya1aj4.png)
Where,
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) is the initial concentration = 1.50 mol/L
is the initial concentration = 1.50 mol/L
![[A_t]](https://img.qammunity.org/2021/formulas/chemistry/high-school/c6se0yk0a5jz0ud2m1a9jh5tv0rk9jx59i.png) is the final concentration = 1/3 of initial concentration =
is the final concentration = 1/3 of initial concentration =
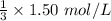 = 0.5 mol/L
= 0.5 mol/L
Rate constant, k = 0.2 L/mol*s
Applying in the above equation as:-
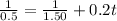
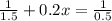
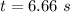
6.66 s will it take for [AB] to reach 1/3 of its initial concentration 1.50 mol/L.