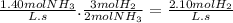Answer:
E. 2.10 M/s
Step-by-step explanation:
Let's consider the following balanced equation.
N₂(g) + 3 H₂(g) → 2 NH₃(g)
The molar ratio of NH₃ to H₂ is 2:3, that is, when 2 moles of NH₃ appear, 3 moles of H₂ disappear.
The average rate of appearance of NH₃ is 1.40 M/s. The average rate of disappearance of H₂ during the same time period is: