Answer
given,
volume of the can = 360 mL = 360 x 10⁻⁶ m³
mass of the full can of pop = 0.369 kg
weight of the empty can = 0.153 N
temperature of water = 20⁰C
weight of the full can
W = m g
W = 0.369 x 9.8 = 3.616 N
Weight of the pop in can
w₂ = W - w₁
w₂ = 3.616 - 0.153
w₂ = 3.463 N
w₂ is weight of the liquid
Specific weight of the liquid
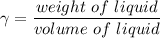
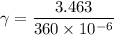
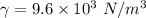
density of liquid
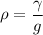
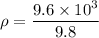
ρ = 979.59 Kg/m³
specific gravity of the fluid
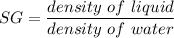
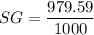
SG = 0.979
Specific gravity of pop is equal to 0.979