Answer:
139.37 g of methanol is present in the given molecules.
Step-by-step explanation:
It is known that 1 mole of any compound contains avagadro's number of molecules. Since here the number of molecules of methanol is given as 2.62 ×
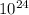 , then the number of moles in them is
, then the number of moles in them is
1 mole = 6.02 ×
 molecules
molecules
So 1 molecule =
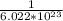 moles
moles
Thus, 2.62 ×
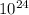 molecules = 2.62 ×
molecules = 2.62 ×
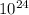 ×
×
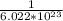 moles = 4.35 moles of methanol.
moles = 4.35 moles of methanol.
As it is known that 1 moles of any compound is equal to the molar mass of that compound. Here we know that 4.35 moles of methanol is present then, the mass is
Molar mass × Number of moles = Mass of methanol
32.04×4.35 = 139.37 g.
So 139.37 g of methanol is present in the given molecules.