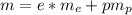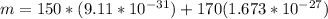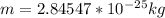According to the statements the number of electrons is 150, then
e = 150
But there is a positive charge of +22e, then the number of protons would be
p = 150+172
If the mass of the electrons is
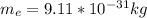
And the mass of the protong is
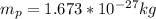
We have that the total mass of the system would be