Answer: Osmotic pressure : increases
Step-by-step explanation:
Osmotic pressure is the minimum pressure which is applied to a solution to prevent the flow of solvent across a semipermeable membrane

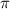 = osmotic pressure
= osmotic pressure
C= concentration in Molarity (number of moles of solute dissolved per liter of the solution)
R= solution constant
T= temperature
Thus as osmotic pressure is directly proportional top concentration, osmotic pressure will increase on increasing the concentration of a nonvolatile solute in water.