Answer:
About 7.9 L.
Step-by-step explanation:
We can utilize the ideal gas law. Recall that:
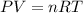
Because the amount of carbon dioxide does not change, we can rearrange to formula to:
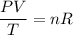
Because the right-hand side stays constant, we have that:
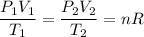
Hence substitute initial values and known final values:
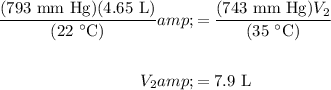
Therefore, the final volume is about 7.9 L.