Step-by-step explanation:
It is known that molar mass of
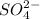 is as follows.
is as follows.
Molar mass =
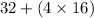
= 96 g/mol
It is given that the concentration of
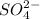 is 1.5 g/L.
is 1.5 g/L.
(a) As 96 g of
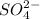 contains 32 grams of Sulfur in 1 L. And, 1.5 g of
contains 32 grams of Sulfur in 1 L. And, 1.5 g of
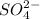 contains x grams of sulfur in 1 L.
contains x grams of sulfur in 1 L.
Therefore, value of x is calculated as follows.
x =
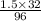
= 0.5 g/L
Hence, the concentration of sulfur is 0.5 g/L.
(b) Now, calculate the molar concentration of sulfur as follows.
Molarity =
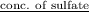
=
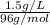
= 0.0156 M
Therefore, the molarity of given sample is 0.0156 M.
(c) Calculate the normality as follows.
Equivalent mass of
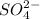 =
=
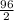
= 48 g/mol
Normality =
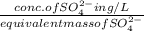
=
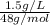
= 0.03125 N
Hence, the normality of given sample is 0.03125 N.