49.491 millimeters of acid is present
Solution:
Given that, chemist is using 351 milliliters of a solution of acid and water
14.1% of the solution is acid
To find: Milliliters of acid present
From given statement,
Solution of acid and water = 351 ml
14.1% of the solution is acid, which means that 14.1 % of 351 ml is acid
Therefore,
Milliliters of acid present = 14.1 % of 351 ml
Here "of" means multiplication
Therefore,
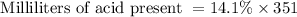
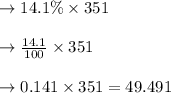
Thus 49.491 millimeters of acid is present