Answer:
Abundance of isotope 79Br=51% and abundance of 81Br=49%
Explanation:
We are given that
Mass of 79Br=78.9183 u
Mass of 81Br=80.9163 u
Average atomic mass of bromine=79.90 u
We have to find the relative abundance of the two bromine isotopes.
Let x be the abundance of 79Br
Then , abundance of 81Br isotopes=1-x
Average atomic mass=Mass of isotope(abundance of isotope)+Mass of other isotope(abundance of other isotope)
Using the formula
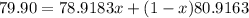
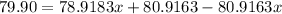
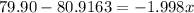
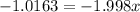
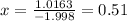
Percentage of isotope with abundance 0.51=
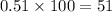 %
%
Abundance of other isotope=1-0.51=0.49
Percentage of other isotope with abundance 0.49=
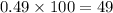 %
%