Answer:
6.67 moles
Step-by-step explanation:
Given that:-
Moles of hydrogen gas produced = 10.0 moles
According the reaction shown below:-
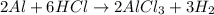
3 moles of hydrogen gas are produced when 2 moles of aluminium undergoes reaction.
Also,
1 mole of hydrogen gas are produced when
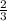 moles of aluminium undergoes reaction.
moles of aluminium undergoes reaction.
So,
10.0 moles of hydrogen gas are produced when
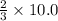 moles of aluminium undergoes reaction.
moles of aluminium undergoes reaction.
Moles of Al needed =
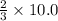 moles = 6.67 moles
moles = 6.67 moles