Step-by-step explanation:
When a strong acid, say
 reacts which a weak base, say
reacts which a weak base, say
 , the reaction is shown below as:-
, the reaction is shown below as:-
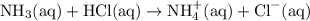
The salt further reacts with water as shown below:-
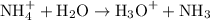
Formation of
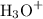 lowers the pH value of the solution as more hydrogen ions leads to less pH.
lowers the pH value of the solution as more hydrogen ions leads to less pH.