1.8 moles of oxygen atoms are present in 30.5 grams of hydrogen peroxide.
Step-by-step explanation:
First we have to convert the given weight of hydrogen peroxide to molar mass of hydrogen peroxide. So for this, we have to divide the given weight with the molecular mass of hydrogen peroxide.
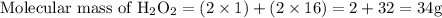
So,
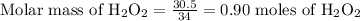
Second step, in this moles, 2 molecules of oxygen are present. Thus 1 mole of Hydrogen peroxide consists of 2 moles of oxygen. Then,
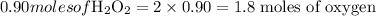
So, 30.5 grams of hydrogen peroxide consists of 1.8 moles of oxygen.