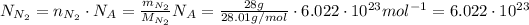Answer:
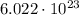 molecules of nitrogen gas
molecules of nitrogen gas
Step-by-step explanation:
In order to convert the mass of a given compound into the number of molecules, we need to:
- identify the number of moles of a given compound dividing its mass by the molar mass,
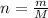 ,
, - multiply the number of moles by the Avogadro's constant which tells us that 1 mole contains
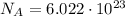 molecules.
molecules.
In this problem:
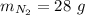
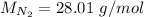
The number of particles is then found by: