Answer:
6.8 moles of NO2=....... g of NO2
= 312.8 g
Step-by-step explanation:
Molar mass : Mass of substance in grams present in one mole of the molecule.
Molar mass of NO2 :
Mass of N + 2(Mass of O)
= 14 + 2(16)
= 14 + 32
= 46 g
Given :
Moles = 6.8 mole
Molar Mass = 46 g
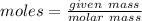
This can also be written as:
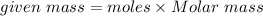
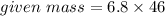
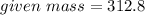
Mass of NO2 In 6.8 moles = 312.8 grams