Answer:
18.8 mL
Step-by-step explanation:
The dilution formula states that the product between the initial molarity and the initial volume should be equal to the product between the final molarity and the final volume, since the number of moles of a substance remains constant in a dilution process:
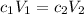
We are given the initial molarity of the stock solution
 , the final molarity of the diluted solution
, the final molarity of the diluted solution
 and the final volume of the diluted solution
and the final volume of the diluted solution
 .
.
Rearranging the expression for the volume of the stock solution, we get:
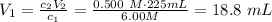
Therefore, 18.8 mL of the stock solution are required.