Answer:
V = 7.95 mL
Step-by-step explanation:
We have in the 50 mL vial the next quantity of CaCO₃:
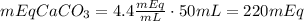
Knowing that in 50 mL we have 220 mEq of CaCO₃, to have 35 mEq we need the following volume of the concentrate (Vc):
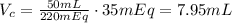
Therefore, for the addition of 35 mEq to the IV order, we need 7.95 mL of the concentrate 4.4 mEq/mL of CaCO₃.
I hope it helps you!