Answer:
The answer to your question is
4C₇H₁₇ + 45 O₂ ⇒ 28 CO₂ + 34H₂O
Step-by-step explanation:
Write the equation
C₇H₁₇ + O₂ ⇒ CO₂ + H₂O
Process
1.- Check if the equation is balanced
Reactants Element Products
7 C 1
17 H 2
2 O 3
As the number of reactants and products is different, we conclude that the reaction is unbalanced.
2.- Write a coefficient "7" to CO₂ and a coefficient of 17/2 to H₂O
C₇H₁₇ + O₂ ⇒ 7CO₂ +
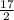 H₂O
H₂O
Reactants Element Products
7 C 7
17 H 17
2 O 51/2
3.- Write a coefficient of 45/2 to the O₂, and multiply all the equation by 2.
4C₇H₁₇ + 45 O₂ ⇒ 28 CO₂ + 34H₂O
Reactants Element Products
28 C 28
68 H 68
90 O 90