Answer:
D. Cd2+
Step-by-step explanation:
The metal ions are classified into four groups which is based on their solubility.
Group II cations are the cations which are based on the solubility of the Sulphides.
Group II (
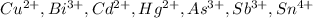 ) cations are the cations which produce very insoluble sulfides.
) cations are the cations which produce very insoluble sulfides.
So, among the given options,
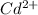 is the only cation which comes in Group II and thus, it will precipitate.
is the only cation which comes in Group II and thus, it will precipitate.