Answer:
The answer to your question is 2.5 billion years
Step-by-step explanation:
The initial amount of Potassium There is X
After 1,25 billion years There will be
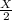
After 1.25 billion years There will be
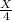
That means that the after 1.25 billion years + 125 billion years there will be one-fourth of the initial amount.
Total time = 2.5 billion years