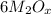Explanation:C
Combustion reaction defined as reaction of compound with the oxygen gas along with release of the large amount heat,.
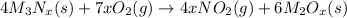
When compound with nitrogen and metal atoms undergoes combustion reaction it gives nitrogen dioxide gas and solid metallic oxide of the metal as a products.
Compound X is nitrogen dioxide.

Compound Y is metal oxide,