Answer:
58.85 g/mol is the atomic mass of the element X.
The element X is cobalt.
Step-by-step explanation:
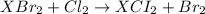
Moles of
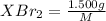 mol
mol
According to reaction, 1 mole of
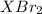 gives 1 mole of
gives 1 mole of
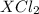 . then
. then
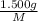 mol of [tex[CBr_2[/tex] will give:
mol of [tex[CBr_2[/tex] will give:
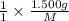 mol=\frac{1.500 g}{M}[/tex] mol[/tex] of
mol=\frac{1.500 g}{M}[/tex] mol[/tex] of
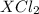
Mass of
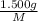 moles of
moles of
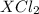 =0.890 g (given)
=0.890 g (given)
Atomic mass of element X =m
M=
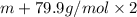
M'=
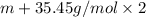
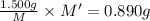
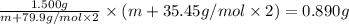
m = 58.806 g/mol
58.56 g/mol is the atomic mass of the element X.
m = 58.806 g/mol ≈ 58.93 g/mol atomic mass of cobalt
The element X is cobalt.