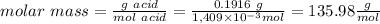Answer:
a.
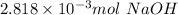
b.
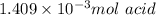
c.
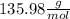
Step-by-step explanation:
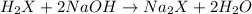
a. To calculate the number of moles of NaOH we need the following definition of molarity:
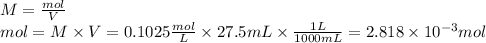
b. As we have a diprotic acid, it means that when the second equivalence point is reached, two moles of NaOH have reacted by one mol of acid, therefore the relation we use to determine the moles of acid is:
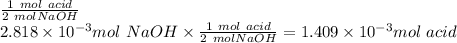
c. Finally, we determine the molar mass as follows: