Answer : The correct option is, (a)

Explanation :
According to the Bronsted Lowry concept, Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
According to the Lewis concept, an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs.
(a)

It is a Lewis-acid because it can accepts electron pairs but not a Brønsted acid because it can not donates hydrogen ion.
(b)
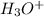
It is not a Lewis-acid because it can not accepts electron pairs but a Brønsted acid because it can donates hydrogen ion.
(c)
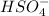
It is not a Lewis-acid because it can not accepts electron pairs but a Brønsted acid because it can donates hydrogen ion.
(d)

It is not a Lewis-acid because it can not accepts electron pairs but a Brønsted acid and a base because it can donates and accept hydrogen ion.
(e)

It is not a Lewis-acid because it can not accepts electron pairs but a Brønsted acid and a base because it can donates and accept hydrogen ion.
Hence, the
 is a Lewis acid but not a Brønsted acid.
is a Lewis acid but not a Brønsted acid.