Answer:
Only C can dissolve in water because its solubility is greater than 0.375 g/mL
C dissolves completely
22.5 g of A remain undissolved in 100 mL of water
2.5 g of B remain undissolved in 100 mL of water
Step-by-step explanation:
Solubility : The amount of salt that can be dissolved in a given volume of solvent at a particular temperature .
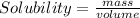
For each salt the solubility of 75 g in 200 mL water is
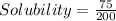
0.375 g/mL
This mean 1 ml of water has 0.375 g of A , B and C
100 mL of water contain = 37.5 g of each salt A , B,C
In the question , the solubility of A , B and C in 100 are given as :
A = 15 g : It means maximum 15 g of A can dissolve in 100 mL
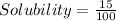 = 0.15 g/mL
= 0.15 g/mL
B = 35 g : It means maximum 35 g of B can dissolve in 100 mL
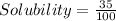 = 0.35 g/mL
= 0.35 g/mL
C = 65 g : It means maximum 65 g of A can dissolve in 100 mL
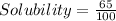 = 0.65 g/mL
= 0.65 g/mL
Since ,Solubility of A and B is less than 0.375 g/mL
So A and B can't dissolve in water at 25°C
A is excess by 37.5 - 15 = 22.5 g
B is in excess by 37.5 - 35 = 2.5 g
Hence 22.5 g of A remain undissolved
Hence 2.5 g of B remain undissolved
Only C can dissolve in water because its solubility is greater than 0.375 g/mL