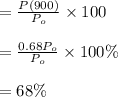Answer:
Percentage of (226Ra) after 900 years is 68%
Explanation:
Let P(t) be the amount of (226Ra) present at any time t
Half life of (226 Ra) = 1599 years
If P₀ is initial amount of (226 Ra) then after 1599 years
P(1599)=P₀/2
Decay i amount of radioactive substance is related to time t as
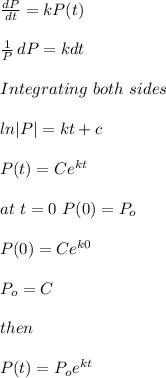
To find value of k
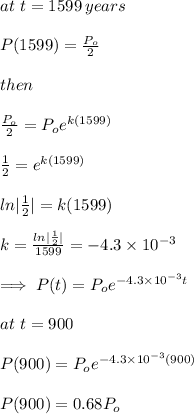
Percentage of radioactive element is:
Amount after 900 years