Answer:
a).Adding hydrogen gas : Forward direction
b) Adding an inert gas
- At constant pressure : Backward direction
- At constant Volume : No change
c) Increasing the volume of the container:Backward direction
Step-by-step explanation:
According to Le-Chatelier's principle = Any disturbance is created in the equilibrium , it will shift the equilibrium in such a way to reduce disturbance.
This disturbance can be : Change in concentration , pressure, temperature , Addition of gas etc.
Temperature : when temperature is increased for endothermic reaction . The equilibrium shift in forward. For exothermic reaction , on increasing temperature ,equilibrium shift backward.
Pressure : On increasing pressure the equilibrium shifts towards less number of gaseous molecules.
Volume : Since volume varies inversely with pressure . It's effect is opposite to that produced by pressure change i.e On increasing Volume the equilibrium shifts towards more number of gaseous molecules.
On Addition of Inert gas : The equilibrium shift toward the side where more gaseous molecules are present.
Concentration : On increasing concentration , the equilibrium will shift such that the substance added get removed .
Haber Process :
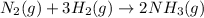
This is exothermic reaction (H = negative)
Molecules of gases are more on left side(reactants) than right side(product)
a) Adding hydrogen gas :
According to Le-Chatelier's principle ,The equilibrium will shift in a direction where the Hydrogen gas is consumed (removed)
Hence Equilibrium shift in forward direction
b) Adding an inert gas : There are two situation
- If inert gas is added at constant pressure: Total Volume is increased, so equilibrium will shift in direction where there is increase in number of molecule(Le-Chatelier's).Here, equilibrium shift in backward direction
- If inert gas is added at constant Volume :No change in equilibrium.This because if inert gas is added pressure will increase but there is no change in moles per unit volume(constant) of gases.
c) Increasing the volume of the container:
If total Volume is increased, the number of molecules per unit volume decrease.In order to maintain the equilibrium constant , the equilibrium shift in direction where there is increase in number of molecule(Le-Chatelier's).
In this case Backward direction.