Answer:
Number of times a one pound (2.2 kg) block of iron can be split in half before it stops being iron
= 84.29 times
Step-by-step explanation:
Atoms : It is the smallest indivisible particle of the matter .
The atom can not be further breakdown .
Here in this question , we have to find the number of atoms (because it is the last possible situation for breaking of atom)
Mole : The quantity of the substance that contain as many particles as present in 12 g of C-12.It is quantity which Avogadro number(N0) of particles
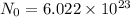
1.First calculate the number of moles of Fe in 2.2Kg Block
Mass of Iron (Fe) = 55.845 amu
Molar mass of Iron = 55.845 g
Given mass = 2.2 kg
1 kg = 1000 g
2.2 kg = 2200 g
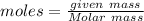
![moles = (2200)/(55.845)</p><p><u><strong>Moles of Fe = 39.39 </strong></u></p><p>2.C<u>alculate the number of particles of Fe in 39.39 moles of Block</u></p><p>[tex]moles = (Number\ of\ Particles)/(Avogadro\ number)]()
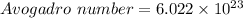
moles = 39.39
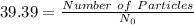
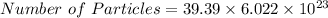
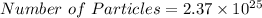
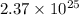 atoms
atoms
3.Calculate Number of time(n), the block is split into -Half
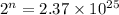
Take log both side ,
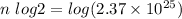
Solve for n, you get
n= 84.29 (convert it to nearest whole number)
n = 84 times