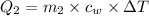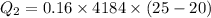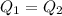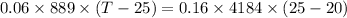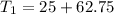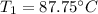Answer:
Step-by-step explanation:
Given
mass of hot metal
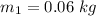
specific heat
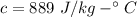
mass of water
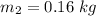
Temperature pf water
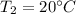
Final Temperature
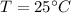
let
 be the temperature of hot metal ball
be the temperature of hot metal ball
Heat lost by heat metal bolt is gained by water in calorimeter
Heat lost by hot metal bolt
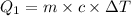
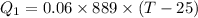
Heat gained by water