Question is complete , here is the complete question:
Which compound produces the greatest number of ions when one mole of it is dissolved in water?
a. NaCl b.
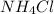 c.
c.
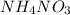 d.
d.
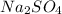
Answer:
The correct answer is option d.
Step-by-step explanation:
Number of ions : N
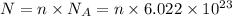
Higher the number of moles of more will be the number of ions.
a) When sodium chloride dissolve in water it gives sodium ions and chloride ions.
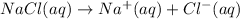
1 mole gives 1 mole of sodium ions and 1 mole of chloride ions.So, 2 moles of ions are produced.
b) When ammonium chloride dissolve in water it gives ammonium ions and chloride ions.
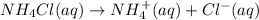
1 mole gives 1 mole of ammonium ions and 1 mole of chloride ions.So, 2 moles of ions are produced.
c) When ammonium nitrate dissolve in water it gives sodium ions and chloride ions.
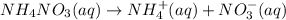
1 mole gives 1 mole of ammonium ions and 1 mole of nitrate ions.So, 2 moles of ions are produced.
d) When sodium sulfate dissolve in water it gives sodium ions and chloride ions.
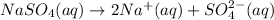
1 mole gives 2 mole of sodium ions and 1 mole of sulfate ions ions.So, 3 moles of ions are produced.
Number of moles of ions dissolving of 1 mole of sodium sulfate in water is maximum and so will be the number of ions.Hence option d is correct.