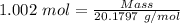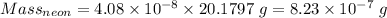Answer:
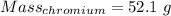
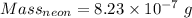
Step-by-step explanation:
Calculation of the mass of chromium as:-
Moles = 1.002 moles
Molar mass of chromium = 51.9961 g/mol
The formula for the calculation of moles is shown below:
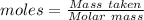
Thus,
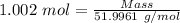
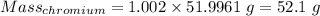
Calculation of the mass of neon as:-
Moles =
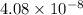 moles
moles
Molar mass of neon = 20.1797 g/mol
Thus,