Answer:
The time required for a 16.00 gram sample of Ge-81 to decay until only 1.00 gram of the sample remains unchanged
30.4 second
Step-by-step explanation:
Half-life = It is the time required by the radioactive substance to becomes half of its original value
For Ge
Half-life = 7.6 s
(it is experimentally determined value)
Calculate how many half-cycles are required to decay 16g of substance to 1 g
(For this divide the original quantity by 2 each time)
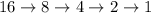
n = number of Half- cycles
Hence total n = 4
1 half - cycles = 7.6 s
4 half-cycles = 4 x 7.6
= 30.4 seconds
Second method :
This can also be calculated by using formula :
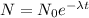
N = Amount of substance after decay
N0 = Original amount of the substance
t = Time required for decay
 = decay constant
= decay constant
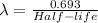
Half -life = 7.6 s
N = 1 g
N0 = 16 g
Insert the values in formula and then Calculate t