Between the temperature points
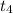 and
and
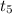 , the molecules are leaving the liquid phase.
, the molecules are leaving the liquid phase.
Step-by-step explanation:
Reading the conversion of states of matter through a graphical representation; we can easily see that the matter is converting from the liquid phase to gaseous phase respective to the temperatures
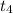 to
to
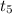 .
.
As the solid is heated at a constant rate at 1 atm pressure, it changes its state from solid to liquid and then liquid to gas consistently.
As the matter crosses the temperature
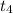 , it leaves it's liquid phase and starts transforming into its gaseous version. It's quite obvious that the temperature will go on increasing until the matter reaches its gaseous state.
, it leaves it's liquid phase and starts transforming into its gaseous version. It's quite obvious that the temperature will go on increasing until the matter reaches its gaseous state.