Step-by-step explanation:
Chemical reaction equation for
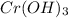 is as follows.
is as follows.
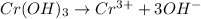
As it is given that pH is 6. So, the concentration of hydrogen ions will be as follows.
pH =
![-log [H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/hggt6ye9u4ukk181i3mah9cn5ybt9okq1d.png)
antilog (-6) =
![[H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/6hb4zpwkhtbq7b9xvc210bz7tsiz8l7l6h.png)
![[H^(+)] = 10^(-6)](https://img.qammunity.org/2021/formulas/chemistry/high-school/ij3nsujbegd40s6nreewqvezoftmknki9p.png)
and
![[OH^(-)] = (10^(-14))/([H^(+)])](https://img.qammunity.org/2021/formulas/chemistry/high-school/3zpsp8wm52d8j0a6x7hobpmi0ykksfm7gt.png)
=
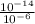
=
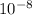
Now, let us assume that the solubility is "s". Therefore,
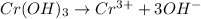
s
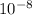
Therefore, calculate the value of
 for this reaction as follows.
for this reaction as follows.
![K_(sp) = [Cr^(3+)][10^(-8)]^(3)](https://img.qammunity.org/2021/formulas/chemistry/high-school/zmmj70jnfu8vtqxiak7skp82gloqaw249z.png)
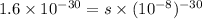
s =
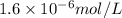
Thus, we can conclude that the molar solubility of
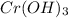 is
is
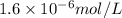 .
.