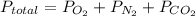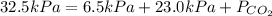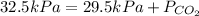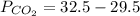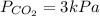Answer:
A gas mixture containing oxygen, nitrogen, and carbon dioxide has a total pressure of 32.5 kPa.
The pressure for oxygen is 3 kPa
Step-by-step explanation:
According to Dalton's Law of Partial Pressure total exerted by the mixture of non-reacting gases is equal to sum of the partial pressure of each gas.
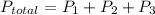
So,
For , a gas mixture containing oxygen, nitrogen, and carbon dioxide has a total pressure:
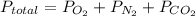
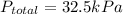
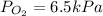
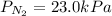
Insert the values in :