Answer:
we can see that the maximum number increases linearly with the atomic number
Step-by-step explanation:
The wave function of a well of infinite potential is
φ (x) = A sin (n π x / L)
Donate A is the amplitude, n the quantum number and L the width of the well
The energy is given by
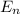 = (h² / 8m L²) n²
= (h² / 8m L²) n²
With n = 1, 2, 3, …
The probability density is
P = φ*φ
P = A² sin² (n π x / L)
To have maximum intensity the function must be ±-1, so the argument must have ±π /2 rad
n π x / L = π / 2
n = L / 2x
Where x varies between zero and L
For case n = 1, it has only one maximum
For n = 2 it has two maximums,
For n = 3 it has three maximums,
For n = 4 it has 4 maximums
we can see that the maximum number increases linearly with the atomic number