Final answer:
The volume of water vapor produced in the combustion of 25.0 liters of O2 for propane combustion is 20.0 liters of H2O following the stoichiometric ratio from the balanced chemical equation.
Step-by-step explanation:
The student is asking about the calculation of the volume of water vapor produced in the combustion of propane gas when a given volume of oxygen gas is consumed. The balanced chemical equation for the combustion of propane is
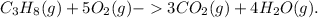
Given that 25.0 liters of O2 are consumed, we can use stoichiometry based on the balanced equation to find the volume of H2O produced. Since the reaction shows that 5 moles of O2 produce 4 moles of H2O, the volume of water vapor produced would be (4/5) × 25.0 liters = 20.0 liters of H2O.