Answer : The equilibrium pressure of
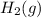 is, 288 torr
is, 288 torr
Explanation : Given,
Initial pressure of
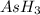 = 392.0 torr
= 392.0 torr
Total pressure = 488.0 torr
The balanced equilibrium reaction is,
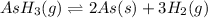
Initial pressure 392.0 0
At eqm. (392.0-2p) (3p)
The expression of equilibrium constant
 for the reaction will be:
for the reaction will be:
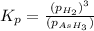
As,
Total pressure at equilibrium = (392.0-2p) + (3p) = 488.0 torr
(392.0-2p) + (3p) = 488.0
392.0 + p = 488.0
p = 488.0 - 392.0
p = 96
Thus, the equilibrium pressure of
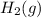 = 3p = 3(96) = 288 torr
= 3p = 3(96) = 288 torr
Therefore, the equilibrium pressure of
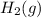 is, 288 torr
is, 288 torr