Answer :
The amount after 1000 years will be, 4.43 grams.
The amount after 10000 years will be, 1.49 grams.
Step-by-step explanation :
Half-life = 5715 years
First we have to calculate the rate constant, we use the formula :
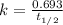
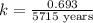
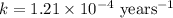
Now we have to calculate the amount after 1000 years.
Expression for rate law for first order kinetics is given by:
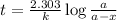
where,
k = rate constant =
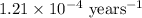
t = time passed by the sample = 1000 years
a = initial amount of the reactant = 5 g
a - x = amount left after decay process = ?
Now put all the given values in above equation, we get
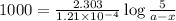
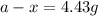
Thus, the amount after 1000 years will be, 4.43 grams.
Now we have to calculate the amount after 10000 years.
Expression for rate law for first order kinetics is given by:
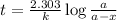
where,
k = rate constant =
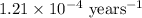
t = time passed by the sample = 10000 years
a = initial amount of the reactant = 5 g
a - x = amount left after decay process = ?
Now put all the given values in above equation, we get
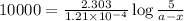
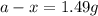
Thus, the amount after 10000 years will be, 1.49 grams.