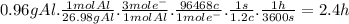Answer:
a. Anode
b. 2.4 hours
Step-by-step explanation:
a.
The initial mass of the electrode is 54.98 g and the final mass is 54.02 g. Since the mass has diminished, the electrode is acting as an anode, in which aluminum is oxidized and the aluminum cation goes into the solution.
Al(s) → Al³⁺(aq) + 3 e⁻
b.
The mass of Al that reacted is 54.98 g - 54.02 g = 0.96 g
We can establish the following relations.
- The molar mass of Al is 26.98 g/mol.
- 1 mol of Al is oxidized when 3 moles of electrons circulate.
- 1 mol of electrons has a charge of 96468 c (Faraday's constant).
- 1 A = 1 c/s
- 1 h = 3600 s
The hours during which the current was applied was: