Step-by-step explanation:
(a) It is known that relation between vapor pressure and mole fraction is as follows.
vapor pressure =
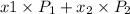
where,
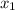 = mole fraction of component one
= mole fraction of component one
 = vapor pressure of component one when pure
= vapor pressure of component one when pure
Also,
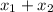 = 1
= 1
Now, putting the given values into the above formula as follows.
vapor pressure =
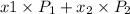
33 =
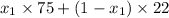
11 =
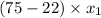
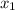 = 0.20754717
= 0.20754717
= 0.21 (approx)
And,
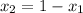
= (1 - 0.21)
= 0.79
Hence, the composition in mole fractions of a solution that has a vapor pressure of 33 torr at
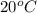 is 0.79.
is 0.79.
(b) According to Raoult's law,
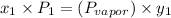
where,
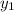 = composition in gas phase
= composition in gas phase
Therefore, calculate the value of
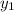 (mole fraction of benzene) as follows.
(mole fraction of benzene) as follows.
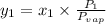
=
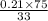
= 0.471698114
= 0.47 (approx)
Thus, mole fraction of benzene in the given vapor is 0.47.