Answer:
The volume occupied by fluorine gas, when the pressure is doubled and temperature is increased to 400 K is 600 ml.
Step-by-step explanation:
As per ideal gas law, the pressure of any gas is inversely proportional to the volume occupied by the gas. Similarly, the volume of the gas is directly proportional to the temperature of the gas molecules. So the ideal gas equation is
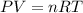 . Here P is pressure, V is volume, n is the no.of moles, R is gas constant and T is temperature.
. Here P is pressure, V is volume, n is the no.of moles, R is gas constant and T is temperature.
So in this case, the fluorine gas occupies 810 mL of volume (
 ) at 270 K temperature (
) at 270 K temperature (
 ) and pressure of about 1 atm (
) and pressure of about 1 atm (
 ). If the pressure is doubled, then the new pressure will be
). If the pressure is doubled, then the new pressure will be
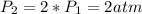 and temperature is increased to 400 K. As the no.of moles will be constant, then
and temperature is increased to 400 K. As the no.of moles will be constant, then
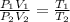
Thus,
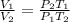
So,
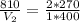
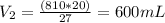
Thus, the new volume occupied by fluorine gas on increase in pressure and temperature is 600 mL.