Answer:
The answer to your question is 4.29 cm³
Step-by-step explanation:
Definition
Density is a measurement that relates the mass of a body to its volume.
Data
density = 3.52 g/ml
mass = 15.1 g
volume = ? cm³
Formula
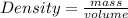
Solve for volume
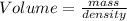
Substitution
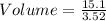
Simplification and result
Volume = 4.29 ml = 4.29 cm³