Step-by-step explanation:
As we know that sum of only total number of protons are also known as atomic number of an atom. And, the sum of total number of protons and neutrons is known as atomic mass of an atom.
Number of protons help in determining the identity of an atom whereas the number of neutrons can be same for two or more number of atoms. But the number of protons will remain the same for a particular atom and for its isotopes.
For example, tex]^{1}_{1}H[/tex] and
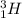 are isotopes and each of them contains 1 proton.
are isotopes and each of them contains 1 proton.
thus, we can conclude that the number of protons in their nuclei would allow you to determine whether two atoms are of the same or different elements.