Answer:
56.0 g
Step-by-step explanation:
Calculation of the moles of
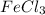 as:-
as:-
Mass = 85.0 g
Molar mass of
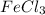 = 162.2 g/mol
= 162.2 g/mol
The formula for the calculation of moles is shown below:
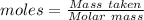
Thus,
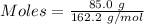
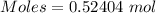
According to the reaction:-
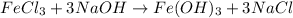
1 mole of
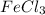 on reaction forms 1 mole of
on reaction forms 1 mole of
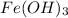
Also,
0.52404 mole of
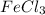 on reaction forms 0.52404 mole of
on reaction forms 0.52404 mole of
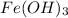
Moles of
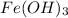 = 0.52404 moles
= 0.52404 moles
Molar mass of
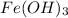 = 106.867 g/mol
= 106.867 g/mol
Mass = Moles*Molar mass =
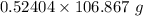 = 56.0 g
= 56.0 g