Answer:
a) 1.740 g of F- must be added to a cylindrical water reservoir
b) Grams of sodium fluoride, NaF, that contain this much fluoride:
3.84 g
Step-by-step explanation:
Step 1. calculate the volume of the tank:
Volume of cylinder =
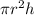 ,
,
Here r = radius of the cylinder = d/2
h = depth = 21.80m
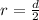
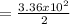
= 168 m
Volume =
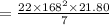

2.Convert ppm to g/m3 and Solve for mass of F-
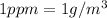
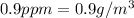
Because both ppm and g/m3 are same quantity .
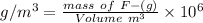
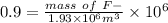
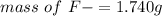
mass of F- required = 1.740 g
3. Apply mole concept to calculate grams of sodium fluoride produced
mass of 1 mole of F2 = 38 g
mass of 1 mole of NaF = 42 g
(from periodic table calculate molar mass)
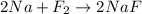
Here 1 mole of F2 produce = 2 mole of NaF
So,
38 g of F2 produce = 2 x 42 g of NaF
38 g of F2 produce = 84 g of NaF
1 g of F2 produce = 84/38 g of NaF
1.74 g F2 produce =
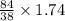
1.74 g F2 produce = 3.84 g of NaF
3.84 g of NaF is produced