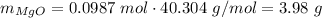Answer:
3.98 g
Step-by-step explanation:
Step 1. Write the balanced chemical reaction. In this case, magnesium reacts with oxygen to produce magnesium oxide:
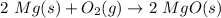
\\
Step 2. Calculate the number of moles of magnesium:
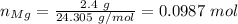
\\
Step 3. Calculate the number of moles of oxygen:
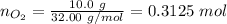
\\
Step 4. Identify the limiting reactant comparing the equivalents. Equivalent of Mg:
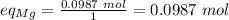
Equivalent of oxygen:
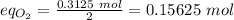
Therefore, Mg is the limiting reactant.
\\
Step 5. According to the stoichiometry of this reaction:
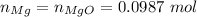
\\
Step 6. Convert the number of moles of MgO into mass: