Answer:
3.14 grams of ammonium thiocyanate must be used to react completely with 6.5 g barium hydroxide octahydrate.
Step-by-step explanation:
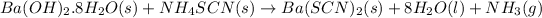
The balance chemical equation is :
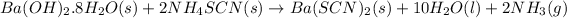
Mass of barium hydroxide octahydrate = 6.5 g
Moles of barium hydroxide octahydrate =
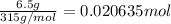
According to reaction, 2 moles of ammonium thiocyanate reacts with1 mole of barium hydroxide octahydrate. The 0.020635 moles of barium hydroxide octahydrate will react with:
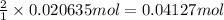
Mass of 0.04127 moles of ammonium thiocyanate;
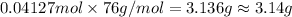
3.14 grams of ammonium thiocyanate must be used to react completely with 6.5 g barium hydroxide octahydrate